Logo and Menu
Event more
- [Max Planck & Nature Day @Yonsei] 2025 MPI–IBS Conference & Nature Session Nov 7, 2025
- [Yonsei-IBS Nobel Forum with Music] 노벨상 수상자 Moungi Bawendi 교수 초청 강연 Oct 24, 2025
- IBS-Yonsei Synergy Program 협약 체결 Oct 17, 2025
- '2025 IBS-Yonsei Nano Medicine Day' 개최 현장 Oct 1, 2025
- 2025 Nano Medicine Day 개최 - 나노바이오메디컬엔지니어링 대학원 입학 설명회 Sep 24, 2025
Seminar more
- [Special Seminar] Prof. Karl Böhringer, Nov/13/2025 "Metasurface Optics for Endoscopy and Hyperspectral Imaging" Nov 28, 2025
- [Special Seminar] Prof. Hyunjoon Kong, Nov/10/2025 "Active Matter Engineering for Biotransport Modulation" Nov 28, 2025
- [Special Seminar] Prof. Oleg Gang, Sep/18/2025 "Programmable Nanoscale Materials" Nov 28, 2025
- [Special Seminar] Prof. Namshik Han, Sep/04/2025 "Revealing the Unseen: AI and Quantum-Inspired Approaches in Novel Target Discovery for High Unmet Needs Through Multi-Omics Integration" Sep 5, 2025
- [Special Seminar] Prof. Ki Bum Lee, Aug/25/2025 "Bio-Inspired Nanomedicine for Precision Control of Cell Fate in Disease Microenvironment" Aug 25, 2025
Research Areas
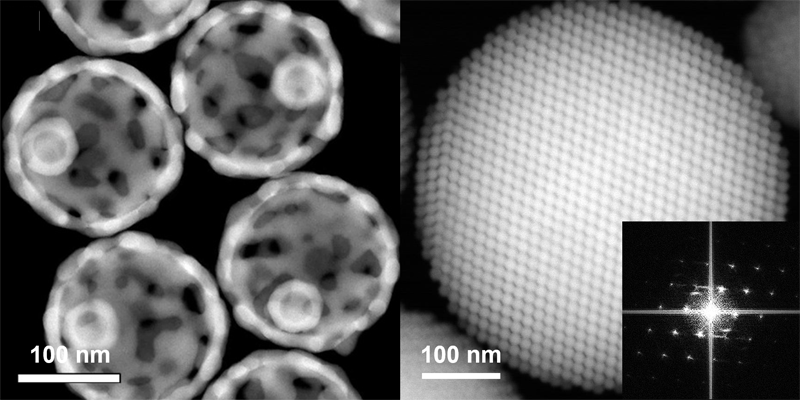
Evolutionary Nanomaterials
This research field focuses on nanoscale designing and engineering of materials to drive advancements in next-generation technologies across electronics, energy, and biomedicine. By exploring unconventional compositions and architectures, researchers aim to develop materials with enhanced or entirely novel functionalities. This innovation-driven approach offers significant potential for addressing complex scientific and biomedical challenges.
Copyright and Address
- Yonsei Advanced Science Institute
- PRIVACY POLICY